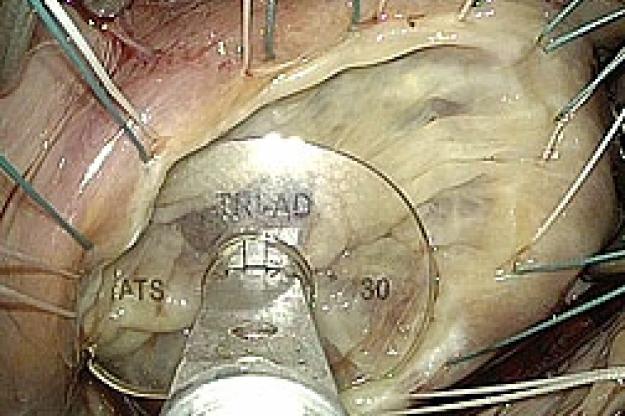
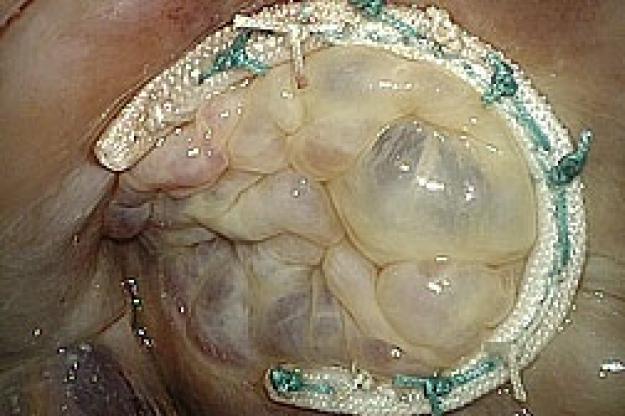
Medtronic Tri-Ad Adams Tricuspid Annuloplasty Ring - First Implantation
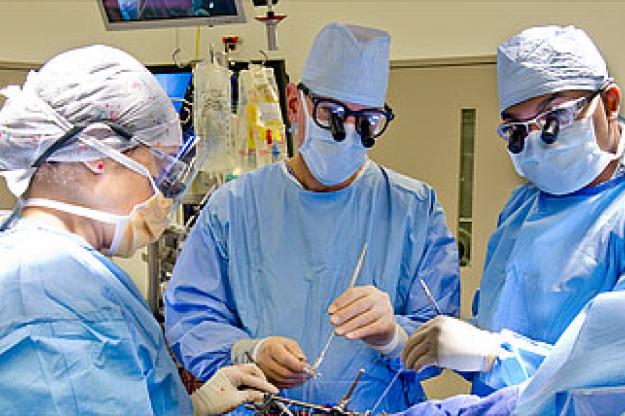
New York, NY, September 09, 2010 -- David H. Adams, MD, Marie-Josee and Henry R. Kravis Professor and Chairman of the Department of Cardiothoracic Surgery at The Mount Sinai Medical Center, has performed the first implantation of the Medtronic Tri-Ad™ Adams Tricuspid Annuloplasty Ring in the United States. Dr. Adams invented the ring, which received recent Food and Drug Administration approval.
“Our understanding of the complex 3-dimensional nature of the tricuspid valve in normal and diseased states has progressed over the past several years due to advances in imaging. We have translated this knowledge into a new concept in tricuspid ring design that combines the characteristics of the currently available tricuspid rings, which up until now have been completely rigid or completely flexible. The Tri-Ad ring has a rigid component to optimally correct the main lesion seen in tricuspid regurgitation, in combination with completely flexible sections to protect delicate tissue while the valve and heart changes its 3-dimensional shapes throughout the cardiac cycle,” said Dr. Adams.
The tricuspid valve lies between the right atrium and right ventricle of the heart. It prevents blood from leaking back into the right atrium during ejection (systole). Tricuspid valve regurgitation occurs when the valve does not close completely, and until recently, “surgical abstention” has been the norm in dealing with functional tricuspid regurgitation, with the assumption that tricuspid regurgitation should resolve once the primary cause (typically mitral stenosis or regurgitation) is eliminated. Annuloplasty rings are specially designed to help restore the tricuspid valve to its normal size and shape (the valve is often enlarged or distorted in a diseased state.) If left untreated, tricuspid valve regurgitation can lead to debilitating symptoms including congestive heart failure and irreversible heart damage. Both American1 and European2 Guidelines for heart valve disease management now emphasize the importance of treating the diseased tricuspid valve at the time of mitral valve surgery.
“We also increased the open side of the new ring to better accommodate the hearts conduction system which is intimately associated with the tricuspid valve. So in addition to a more advanced physiologic repair concept, the Tri-Ad ring design will also protect against damage to the electrical system of the heart,” noted Dr. Adams.